FDA Questions and Answers about Withdrawal of Fenfluramine Pondimine and Dexfenfluramine Redux wwwfdagov July 7 2005. Johnson and Johnson which makes a.

Fda Recall Watch Mcintyre Law Pc
Pfizer submitted a request for full approval to the FDA in May.

Fda approved vaccines that were recalled. How is a vaccine recalled. Biden Praises Pfizer-BioNTech Vaccine Approval President Biden lauded the final approval of the Pfizer-BioNTech vaccine by the Food and Drug. The FDA rarely has recalled vaccine lots for concerns such as mislabeling contamination during production and potential manufacturing problems at a production plant.
Its been almost 7 months since the Food and Drug Administration FDA issued the first emergency authorization of a. For a vaccine to be FDA approved scientists must gather enough data through clinical trials in large numbers of volunteers to prove it is safe and effective at protecting people. Federal regulators have decided to scrap about 60 million doses of JJs Covid vaccine produced at a troubled Emergent BioSolutions plant in Baltimore.
The other mRNA vaccine manufacturer Moderna tendered its request in early June. 368 Zeilen FDA approved new vaccine Cervarix GlaxoSmithKline for the prevention of cervical cancer. Soldiers had sued claiming a mandatory anthrax vaccine.
Only once before has the FDA given a vaccine this lesser standard approval of an EUA but it was in an unusual circumstance. Prior to Monday the PfizerBioNTech vaccine had received the FDAs approval under the agencys Emergency Use Authorization authority which some vaccine opponents used as an argument against. 8 10 While clinical trials provide important information on vaccine safety the data are somewhat limited because of the relatively small number hundreds to thousands of study participants.
Whenever a vaccine lot is to be recalled FDAs role is to oversee a manufacturers strategy and help ensure the recall. FDA Raplon Rapacuronium Bromide wwwfdagov Mar. FDA recognizes the gravity of the current public health emergency and the importance of facilitating availability as soon as possible of vaccines to prevent COVID-19 - vaccines that the public.
Valdecoxib is an NSAID that the FDA later determined worked no better than other NSAID pain medications on the market. Some of them lumiracoxib rimonabant tolrestat ximelagatran and ximelidine for example were approved to be marketed in Europe but had not yet been approved for marketing in the US when side effects became clear and their developers pulled them from the market. So glad the FDA could join us six months late Daily Wire Editor Emeritus Ben Shapiro asked Monday following news that the FDA has finally approved Pfizers coronavirus vaccine after Americans have already received more than 200 million doses.
Experts say full approval may help increase vaccination rates. Coronavirus COVID-19 Update. Pfizer and BioNTech which developed one of the three COVID-19 vaccines available in the US in May completed their application for full FDA approval for use in.
The FDA authorized the use under the emergency use authorization EUA for the Janssen COVID-19 vaccine of an additional batch of vaccine. This list is not limited to drugs that were ever approved by the FDA. WHO and ACIP issued recommendations on the use of H1N1 influenza vaccines.
The FDA rarely issues a recall and if safety is a concern the recall is immediate. The drug was recalled for adverse heart effects including death heart attack and stroke as well as increased risk for serious skin reactions such as epidermal necrolysis erythema multiforme and Stevens-Johnson syndrome. The FDA granted full approval to Pfizers Covid-19 vaccine making it the first vaccine to be fully approved in the US.
To boot the drug had the potential to cause. LSD were never approved for. FDA approved new indication for gardasil to prevent genital warts in men and boys.
FDA approved four vaccines against the 2009 H1N1 influenza virus. Some drugs in this list eg. The approval comes in a week prior to federal health officials earlier estimates that the agency would complete its review by Labor Day.
FDA Recalling the Omniflox Temafloxacin Tablets June 5 1992. Robyn BeckAFP via Getty Images FILE The Food and Drug Administration on Monday granted full approval of the Pfizer COVID-19 vaccine becoming the first covid-19 vaccine to transition from an emergency authorization status to full FDA approval. The manufacturer contacts vaccine distributors and healthcare facilities who might have purchased the vaccine to inform them of the suspected problem.
Vaccine recalls or withdrawals are almost always initiated voluntarily by the vaccine manufacturer.
Fda Warning About J J Vaccine Creates More Challenges For Fighting Covid Crisis
Pfizer Biontech And Moderna Covid 19 Vaccines Establish Recall Responses To Reinfection
Ub Med School Doctor Fda Approval Expected By September For Covid Vaccines Wgrz Com
Pfizer Biontech And Moderna Covid 19 Vaccines Establish Recall Responses To Reinfection
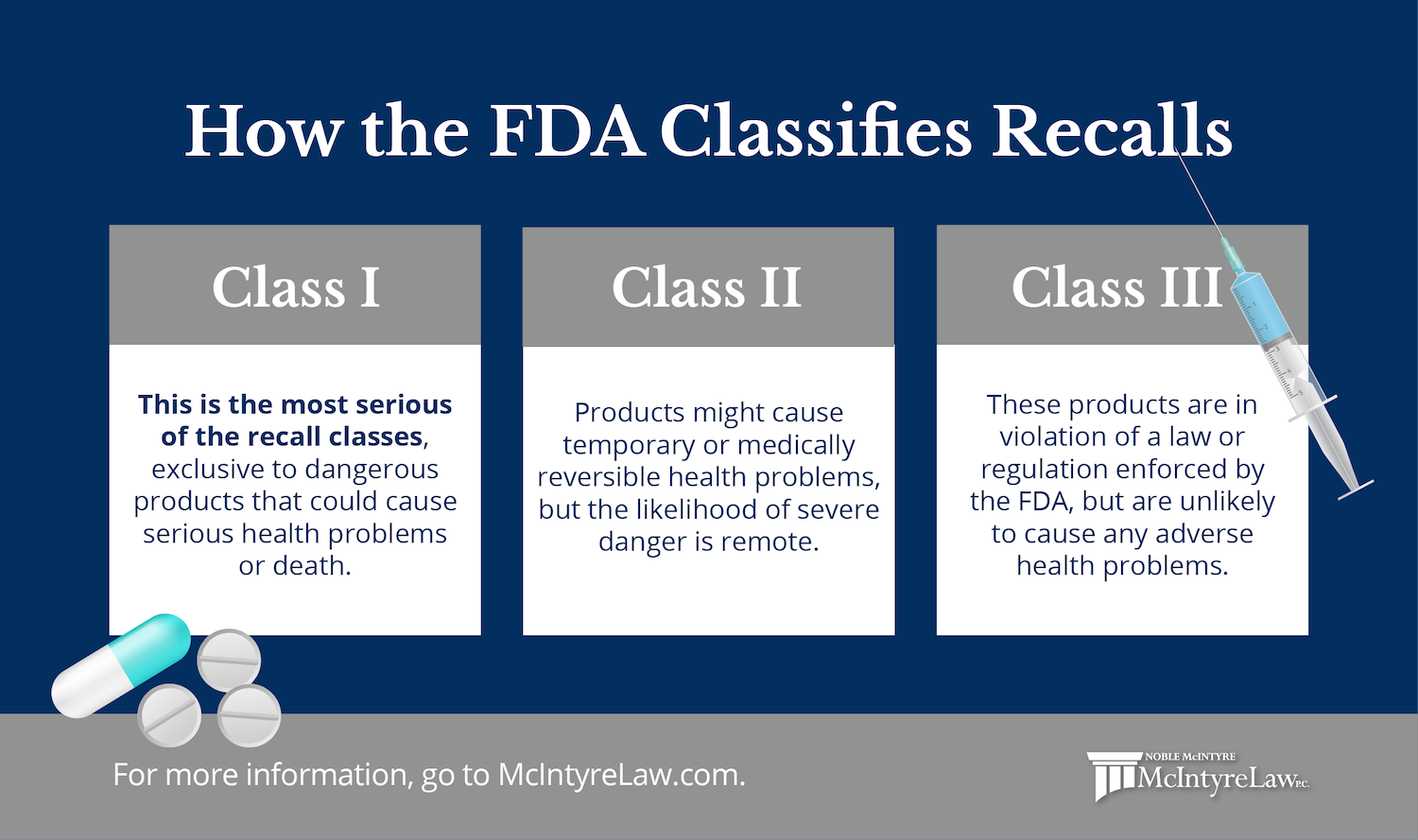
Fda Recall Watch Mcintyre Law Pc

Covid Surges Emerge Fda Warns About Chantix Lions Tigers Bears Get Vaxxed Medpage Today

Overview Of The Fda S Drug Recall Process

Johnson Johnson Recalls Sunscreen Fda Adds Warning To Janssen Covid 19 Vaccine About Rare Guillain Barre Syndrome

Fda Analysis Supports Emergency Use Of Pfizer Covid 19 Vaccine Shots Health News Npr

Fda Grants Priority Review Status To Pfizer Vaccine
Rushing Full Approval For Covid Vaccines Could Backfire Wsj

Verify Covid 19 Anti Vaccine Meme Checklist Is Misleading Abc10 Com

Pfizer S Covid 19 Vaccine Expected To Receive Full Fda Approval By Labor Day Newsnation Now
Did The Fda Recall All Covid 19 Pcr Tests Healthcare Packaging
Https Www Fda Gov Files About 20fda Published The Road To The Biotech Revolution Highlights Of 100 Years Of Biologics Regulation Pdf